
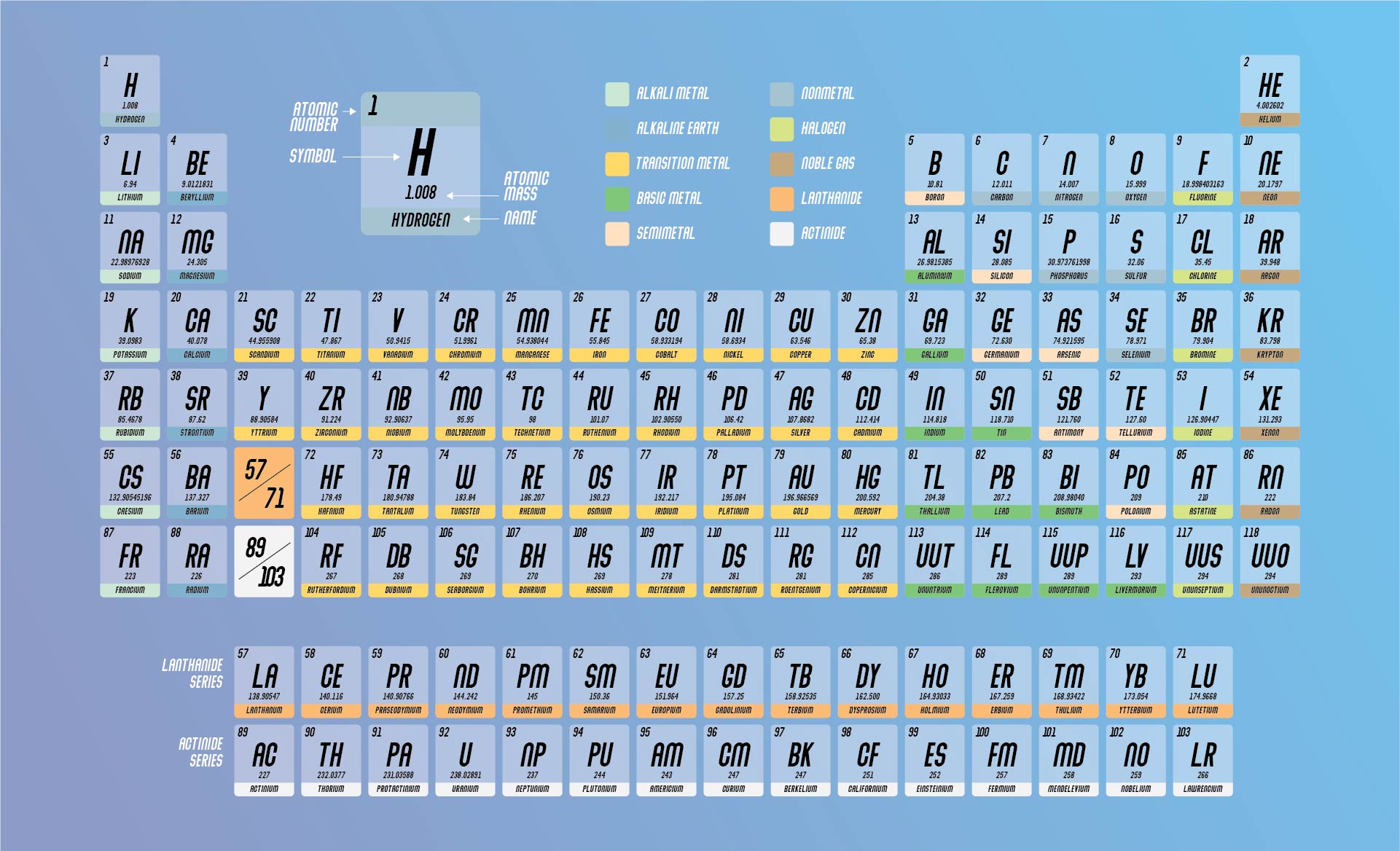
For example, the elements in group 18 are called ‘ Noble Gases’. For example, the elements of group 18 are all gases that do not readily react with other elements. The elements within a group usually have similar properties. The groups on the periodic table are numbered from 1 to 18. But the principles of groups and periods still exist in our modern version. Mendeleïev’s table was rearranged to give us the format that we use today. Mendeleev predicted the missing elements would have the same properties as the other elements in the same column.ĭiscoveries led to many new elements during the 1900s. Those gaps were for undiscovered elements. He believed that some elements had not yet been discovered. The columns are known as groups (or families). All elements in the same period have the same number of electron layers, also called shells. Then he organized rows and columns to highlight elements with similar chemical properties. Mendeleev organized the table so that the elements from low to high atomic mass. By subtracting 20 from 40 (rounded), we determine that the average number of neutrons is 20. For example, calcium has an atomic number of 20 and an atomic mass of 40.078 u. You need to subtract the number of protons (atomic number) from the atomic mass.
Getting the average number of neutrons for an element is easy. Those, with up to 7 neutrons, do not occur in nature. Scientists have also created other highly unstable isotopes of hydrogen. The three naturally-occurring isotopes of the hydrogen atom (©2021 Let’s Talk Science).
:max_bytes(150000):strip_icc()/PeriodicTableValence-58b5d8f95f9b586046df59fb.jpg)
The most common isotope, protium, has no neutrons! Deuterium has one neutron and tritium has two neutrons. For example, hydrogen has three naturally occurring isotopes. Isotopes of an element have the same number of protons, but different numbers of neutrons. The atomic mass is a decimal number because it is an average of the masses of the various isotopes of an element. This is usually given at the bottom of an element’s entry in the periodic table. The atomic mass of an element refers to the average mass of an element in unified atomic mass units (short form u). The number of protons influences the chemical behaviour of an element. For example, any atom that contains exactly 20 protons in its nucleus is an atom of calcium. The atomic number is equal to the number of protons in an atom's nucleus and determines which element an atom is. The number above the atomic symbol represents the atomic number. Some people attribute the first categorization to Alexandre-Emile Béguyer de Chancourtois.Ĭlose-up of how an element is presented in the periodic table (©2021 Let’s Talk Science). In Germany, Lothar Meyer also came up with an almost identical table later the same year. Dmitri Mendeleev, a Russian scientist, is usually credited with creating the first periodic table in 1869.

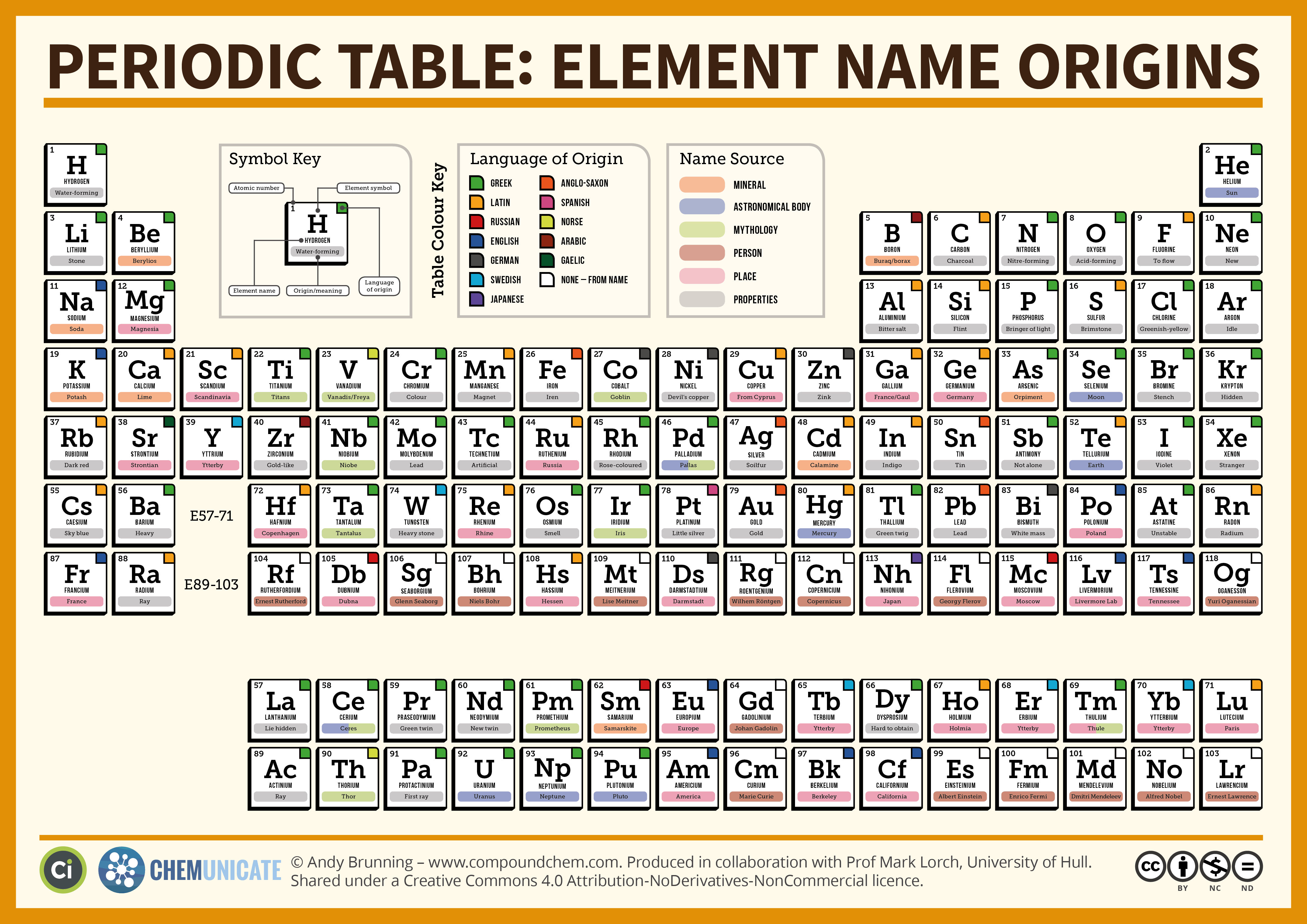
The periodic table of the elements is a visual and logical way to organize all elements. This is because its natives gave silver gifts to the first European conquerors. Its name is from the latin name of silver, argentum. Other elements have been named for places, such as: ElementĪrgentina got its name from an element. Pierre and Marie Curie (research in radioactivity)ĭmitri Mendeleev (creator of the first periodic table) Greek word neos meaning ‘new’ or ‘different’Īs well for famous scientists such as: Element Greek word hydrargyrum meaning ‘silver water’ Many of the elements have their name or their symbol based in Latin or Greek. Some elements have even been discovered within your lifetime! Naming the Elements Scientists discovered many others during the 18th and 19th centuries. People have known about some elements such as gold, silver, iron, mercury and tin for hundreds of years. Matter that is composed of only one type of atom is called an element.


 0 kommentar(er)
0 kommentar(er)
